What Is the Hybridization State of Si in H3si Sih3
Enter the numbers corresponding to the correct geometry in the boxes next to the formula. Start your trial now.
Solved What Is The Hybridization State Of Si In Sih 4 Sp 2 Chegg Com
What is the hybridization state of Si in SiH4 and in H3Si-SiH3.

. Therefore 442 4 Hence its sp3 hybridized. A sp2 B sp3d C sp3 D sp 23. Chemistry questions and answers.
D trigonal planar trigonal planar. Since all the valence electrons participate in hybridization then it has no lone pair of electrons in it. O has been changed from sp 3 to sp 2.
Si belongs to the carbon family Z 14. What is the hybridization of silica in silicon dioxide. Ne3s23p2SiO2 forms a covalent network in.
Electron Domain Geometry Molecular Geometry a NH4. Describe the hybridization state of phosphorus in phosphorus pentabromide. In the hydrocarbon a What is the hybridization at each carbon atom b How many Ï bonds are there in the molecule.
What is the hybridization state of Si in SiH4 and in H3Si-SiH3. For full functionality of this site it is necessary to enable JavaScript. What is the change in hybridization if any of the Al atom in the following reaction.
The hybridization state of Si in SiH4 is sp3 hybridized and in H3Si SiH3 it is also sp3 hybridized 1034. The hybridization of SiH 3. In S i H 4 the silicon atom is the central atom which forms four sigma bonds with hydrogen atoms.
First week only 499. Up to 256 cash back What is the hybridization of Si in SiH. What is the hybridization state of Si in SiH4 and in H3Si-SiH3.
Paramagnetic properties of oxygen molecule can be properly. The hybridisation can be calculated as. Now we know that if the steric number is 4 then hybridization is s p 3 and.
Starting with Lewis structure determine the number and type of hybrid orbitals necessary to rationalize the bonding in SiH 4. Solution for What is the hybridization state of Si in SiH4 and in H3Si SiH3. Here are the instructions how to enable JavaScript in your web browser.
The angle between two sp3 hybrid orbitals is. This results in a pi bond between the d and p orbitals which is called a d π -p π bond. Predict the geometries of the following ions.
Consider the reaction BF 3 NH 3 F 3 B NH 3. Check out a sample QA here. What is the hybridization state of Si in SiH4 and in H3Si-SiH3.
Step 1 of 5. Hybridization is the concept of mixing of similar shape and energetic atomic orbitals that form new hybrid orbitals with different energies shapes than the atomic orbitals. Answer to What is the hybridization state of Si in SiH4 and in H3Si-.
Its actually due to the back bonding basically silicon is an element in the 3rd period and nitrogen is an element in the 2nd period so here silicon will contain empty orbitals of 3d and whereas nitrogen has lone pair of electrons so therefore back bonding occurs so while calculating the steric number in order to calculate the hybridization the lone pair on central. Of valence electrons no. Students also viewed these Organic Chemistry questions Give the hybridization state of each carbon in the following Compounds.
NNN A sp3d B sp2 C sp3 D sp 29. S i has vacant d orbital which can take part in back bonding ie P Π d Π back bonding by accepting back the lone pair of electrons of O atoms to its vacant d orbital. What of hybrid orbitals is formed when one s three p and one d orbitals are mixed.
Want to see the full answer. B trigonal bipyramidal t-shaped. Of monovalent atoms attached 2 So here Si has 4 valence electrons and H are 4 monovalent.
The hybridization of Si in SiO2 is sp3Si. Answer 1 of 2. It valance orbital diagram is.
Si has vacant d orbital which can take part in back bonding ie P Π. What is the hybridization state of the central N atom in the azide ion N3-. Solution for What is the hybridization of the central atom in a SiCl4b HCN c SO3 d.
100 6 ratings for this solution. The hybridization of S i H 3 2 O has been changed from s p 3 to s p 2. Lawson Company produces a product where all manufacturing inputs are applied uniformly.
The shape of trisilylamine H 3 Si 3 N is planar this is due to the fact that one of the empty d orbitals in Silicon interacts with the p orbital of Nitrogen containing the lone pair of electrons. So on using the formula of steric number rule which is given as-. Hybridization state of Si in H3Si-SiH3.
For unlimited access to Homework Help a Homework subscription is required. What is the hybridization state of Si in SiH4 and in H3Si SiH3. What is the hybridization of Si in SiH4 and in H3Si-SiH3.
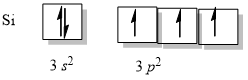
What is the hybridization state of Si in SiH4 and in H3Si SiH3. Step 1 of 4. The ground state electron configuration of the Si atom is Ne 3 s2 3 p2.
100 39 ratings for this solution. The formation of this pi bond results in a planar. The Lewis structure of SiH 4 is.
The company produced the following physical flow schedule for MarchUnits to account forUnits in BWIP 40 percent complete 15000Units started 35000Total units to account for 50000Units.
Solved What Is The Hybridization State Of Si A In Sih4 And B Chegg Com
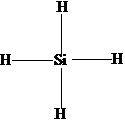
Solved What Is The Hybridization State Of Si A In Sih4 And B Chegg Com

No comments for "What Is the Hybridization State of Si in H3si Sih3"
Post a Comment